Joshua D. Schiffman and Matthew Breen
Abstract
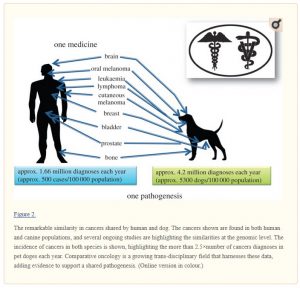
Over 1.66 million humans (approx. 500/100 000 population rate) and over 4.2 million dogs (approx. 5300/100 000 population rate) are diagnosed with cancer annually in the USA. The interdisciplinary field of comparative oncology offers a unique and strong opportunity to learn more about universal cancer risk and development through epidemiology, genetic and genomic investigations. Working across species, researchers from human and veterinary medicine can combine scientific findings to understand more quickly the origins of cancer and translate these findings to novel therapies to benefit both human and animals. This review begins with the genetic origins of canines and their advantage in cancer research. We next focus on recent findings in comparative oncology related to inherited, or genetic, risk for tumour development. We then detail the somatic, or genomic, changes within tumours and the similarities between species. The shared cancers between humans and dogs that we discuss include sarcoma (osteosarcoma, soft tissue sarcoma, histiocytic sarcoma, hemangiosarcoma), haematological malignancies (lymphoma, leukaemia), bladder cancer, intracranial neoplasms (meningioma, glioma) and melanoma. Tumour risk in other animal species is also briefly discussed. As the field of genomics advances, we predict that comparative oncology will continue to benefit both humans and the animals that live among us.
1. Introduction
Comparative oncology is a quickly expanding field that examines both cancer risk and tumour development across species. Characterized by interdisciplinary collaboration, its goal is advancement of both human and animal health. Nowhere has this been more evident than in the investigation and comparison of canine and human tumours. The study of naturally occurring cancers in the domestic dog provides a suitable model for advancement of the understanding, diagnosis and management of cancer in humans [1–4]. There are over 70 million pet dogs in the USA, residing in over 40 million households [5,6]. With over 100 million vet visits each year in the USA, our dogs provide a powerful resource for closely monitored health data. Canine cancers occur spontaneously, and have similar clinical presentation and pathophysiology to equivalent human cancers. As such, they closely parallel the natural progression of human cancer to a greater extent than is observed in induced cancer animal models [7–9]; genomic analysis of canine tumours has revealed shared features between both species, providing fresh insight into the genetic basis of tumour development [10–12]. Additionally, society’s practice of dog breeding has unwittingly created a high-risk model for breed-specific disease due to consanguinity and inbreeding [13,14]. The restricted genetic variation in many purebred dog breeds allows for easier identification of the genetic basis of disease, including cancers, and the shorter lifespan of dogs facilitates timely and efficient evaluation of new approaches to cancer diagnosis, treatment and prevention. This review will discuss the domestic dog as a model for comparative oncology, with a focus on both inherited risk and tumour genomics, along with a brief assessment of other species relevant to the field of study.
(a) On the origin of dogs (and their cancer risk)
While there are probably over 400 breeds of dog recognized worldwide, the number of breeds recognized by the dog fancy in different countries varies. For example, in the USA the American Kennel Club (AKC) now recognizes 184 breeds of dog (including 21 varieties) and the United Kennel Club (UKC) recognizes over 300 breeds. The characteristics of each dog of a specific breed have been refined and maintained to meet the stringent breed standards and be considered a contender for selection as a champion.
Over 15 000 years ago, man’s relationship with the dog was quite different, being one in which dogs were selected primarily for their function. Dogs helped humans to survive, using their fast pace to aid in hunting of animals and their strength and boldness to serve as protectors. Over the course of subsequent millennia, the relationship between man and dog continued to be one of co-dependency. Around the time of the industrial revolution, we started to breed dogs as much for their form as their function, in an industrializing society. In just the past few hundred years, intensive selection and breeding of dogs for key desirable traits resulted in the development of what we regard today as the major characteristics of the breed. When considered as a species, the level of genetic variation sampled across all dog breeds today is perhaps as extensive as for human populations [15]. In individual pure breeds, however, the level of genetic diversity is now variably restricted [16]. The process of breed formation over just the past 2–3 centuries has been estimated to have resulted in sevenfold greater reduction in genetic diversity than did the thousands of years of early domestication [17]. This is compounded further by the use of popular sires and gene pool decline during the twentieth century. Since many of the key phenotypes are characteristics of the particular breed, their presence in the breed had been positively selected, resulting in high frequency of the genes that cause these specific phenotypes. With such intense selection, it is perhaps not surprising that there are now over several hundred inherited diseases recognized in dogs. While some diseases have simple inheritance patterns, others, including cancers, are likely to be more complex. The genetic background of some purebred dogs may predispose the breed to a higher risk for specific cancers, or cancers in general. It is this increased risk of cancer in purebred dogs that may be leveraged to accelerate the process of cancer gene discovery from a comparative perspective.
The histological and clinical presentation of numerous canine cancers closely parallels that of the corresponding cancers in humans. The extended lifespan of the dog, combined with the shared environment and development of spontaneous cancers, places the dog in a unique position to better reflect cancer development and progression than traditional rodent models. As is the case for human and rodent models, the advancement of genomic technologies and the development of canine custom reagents and resources have facilitated studies of both somatic and inherited genomic variation in the domestic dog. As discussed in §2, canine cancers share evolutionarily conserved genomic changes that are found in their human counterparts. Man’s best friend is already providing scientists with an opportunity to generate data beneficial to both species [3,4,10,11,18,19].
(For the rest of the article, including illustrations and references, please follow this link.)