Maxx Chatsko, The Motley Fool
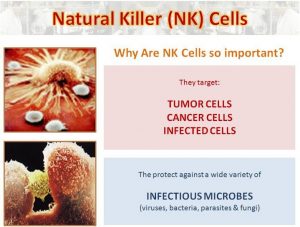
Viruses, infectious microbes, and cancers might invoke fear in healthy humans, but the foreign invaders and mutants have a boogeyman of their own: natural killer (NK) cells. These immune cells certainly live up to their ominous name.
NK cells lurk in your tissues and bloodstream, are the first to respond to molecular signals from damaged or stressed tissues, and mobilize the rest of your immune system — including T cells, B cells, and the like — to get to work. Researchers have shown that NK cells can still wreak havoc on cancer when other immune cells are disabled, they’re the tumor-busting force behind the health benefits of exercise, and long-term survival rates for some of the deadliest cancers appear to be influenced by the activity of NK cells and the genes that control them.
That’s led to a healthy amount of research into NK immunotherapies, which could boast significant advantages over first-generation immunotherapies based on chimeric antigen receptor T-cells (CAR-T). Unfortunately, biopharma companies have been largely unable to replicate the inherent seek-and-destroy capability of NK cells in early stage clinical trials. Can they ever live up to the hype?
Why develop NK immunotherapies?
NK cells were first explored as immunotherapy candidates in the 1980s, but human understanding of biology and biomanufacturing kept a tight lid on the potential. Decades later, the field is finally catching up. That’s important because, on paper at least, immunotherapies based on NK cells boast several noteworthy advantages over CAR-T therapeutics.
For instance, NK cells and CAR-NK therapeutics can be produced from inherently allogeneic (“off-the-shelf”) cell lines, which means a single cell line or donor could produce an infinite number of therapeutic doses. CAR-T medicines have to be strictly matched from donor to patient, or engineered with gene editing platforms such as CRISPR, which increases manufacturing steps and drives drug prices higher.
Allogeneicity also means engineered NK cells don’t trigger negative immune responses in patients, such as cytokine release syndrome, which has led to multiple clinical holds and patient deaths for trials involving CAR-T therapies. That should allow each dose to drive a more durable response, and could allow patients to receive multiple doses of NK therapies, which could significantly improve clinical outcomes and survival rates compared to CAR-T therapies.
Perhaps most important, NK cells can identify and target cancer cells with or without CARs. That should significantly lessen the risk that cancers develop resistance to treatment and reemerge in patients stronger than before, which is possible with molecular antibodies, checkpoint blockade (CPB) drugs, and CAR-T medicines. It could also create the opportunity to combine immunotherapies to create a one-two punch that sends cancer cells to their death.
From The Motly Fool